At last, we introduce the diagnostic efficiency in the serum β-glucan assay and its contribution to the early prognosis of clients at risk for invasive fungal illnesses and fungal septicemia.
in vivo pyrogen testing. When the in vitro pyrogen testing is completed outside a living procedure (i.e. in a very test plate or card) utilizing antigenic substances, the in vivo pyrogen testing is Typically performed inside of a living method like in a laboratory mouse or rabbit. The
The Limulus amebocyte lysate (LAL) assay was initial produced inside the 1960s and commercialized to be a Guess within the U.S. within the seventies. The LAL assay is formulated utilizing specialised blood cells, or amebocytes, obtained from your blue blood of Atlantic horseshoe crabs.
If a test is performed at the MVD and an out-of-specification (OOS) test final result occurs that can't be attributed to testing mistake, the large amount must be turned down. [13] All testing strategies, which includes those for retesting inside the earlier mentioned boundaries, need to be specified in progress in published conventional working methods authorised by the business’s high-quality Manage unit.
Completeness and Clarity of Answer— Constitute the answer as directed within the labeling equipped by the company with the sterile dry dosage type.
The pyrogenic principles of bacteria Primarily Gram detrimental microorganisms are frequently attributable to some warmth-steady substances secreted by these organisms; and which if located in parenteral medications could induce fever (an increase in your body temperature from the host getting the medication). This phenomenon necessitates the necessity to consistently test and detect the presence of pyrogens in intravenous drugs along with other parenterals so which the batch in the products made up of fever-inducing brokers may be stopped from reaching most people.
amoebocyte lysate (LAL). The amoebocytes are the most crucial factors of the blue haemolymph of the Horseshow crab; and click here it truly is what's accountable for the coagulation of your haemolymph in Limulus
The Pharmacy bulk offer is for use only in an appropriate get the job done location such as a laminar flow hood (or an equivalent clear air compounding place).
Obtain aseptically containers which are freed from detectable endotoxins in depyrogenated glassware equipment.
Monograph limits may also not account for latest product strengths or dosage regimes; these should also be checked utilizing the calculations suggested while in the benchmarks.
Carry out the test utilizing a group of 3 rabbits. Preparing of the sample. Dissolve the substance below evaluation in, or dilute with, pyrogen-totally free saline Resolution or other Alternative more info prescribed in the monograph. Warm the liquid under evaluation to roughly 38.5° just before injection.
Soon after coagulation and subsequent gelling, the resulting gel is assumed to incorporate bacterial infections while in the animal's semi-closed circulatory procedure.[3] Contemporary analysis in the lysate has led to idea of This technique of cascade, with multiple enzymes Doing the job in sequence to create the gel. The entry level of endotoxin-induced clotting is Limulus clotting variable C.[4]
. CONSTITUTED Remedies Dry solids from which constituted answers are geared up for injection bear titles of the shape [DRUG] for Injection. Mainly because these dosage types are constituted at time of use through the health care practitioner, tests and standards pertaining to the solution as constituted for administration usually are not included in the individual monographs on sterile dry solids or liquid concentrates.
Favourable Handle needs to be included to confirm that it is appropriate to utilize the parameters of a previous (archived) typical curve to determine endotoxin concentrations.
 Danny Tamberelli Then & Now!
Danny Tamberelli Then & Now! Danielle Fishel Then & Now!
Danielle Fishel Then & Now! Erik von Detten Then & Now!
Erik von Detten Then & Now!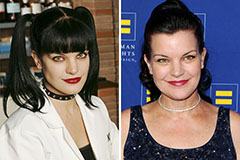 Pauley Perrette Then & Now!
Pauley Perrette Then & Now! Peter Billingsley Then & Now!
Peter Billingsley Then & Now!